Diagram n2 mo orbital molecular diagrams electrons chemistry two electron lie together explain determined different improve answer stack sponsored links 9.10: molecular orbital theory predicts that molecular oxygen is Orbital molecular diagram nitrogen theory ethyne n2 orbitals structure carbon state molecule mot atomic monoxide diagrams chemistry energy level fluorine
mathematics - Origins of molecular orbital diagrams? - History of
Drawing molecular orbital diagrams Orbital molecular diagram cl2 s2 molecule mot unpaired orbitals bond electron bonding draw molecules c2 mo energy theory valence electrons Molecular orbitals
Chapter 6.5 delocalized bonding and molecular orbitals
Orbital delocalized homo orbitals lumo bonding 2s moleculeMolecular orbital theory Orbital molecular chemistry orbitals atomic two theory bond wave axis mo atoms sigma overlap combining antibonding between along internuclear whichOrbital energy theory molecular diagram orbitals mo two atomic 1s rules principle these first fill stable.
Give the molecular orbital theory diagram for the formation of n2 molMolecular orbitals orbital bonding atomic pi atoms delocalized diatomic chem formation libretexts antibonding chemical combine molecules readings formed axis lobes Molecular orbital diagramOrbital molecular diagram b2 theory energy electrons libretexts unpaired chem chemistry bonding pageindex predicts figure two deki api revision.
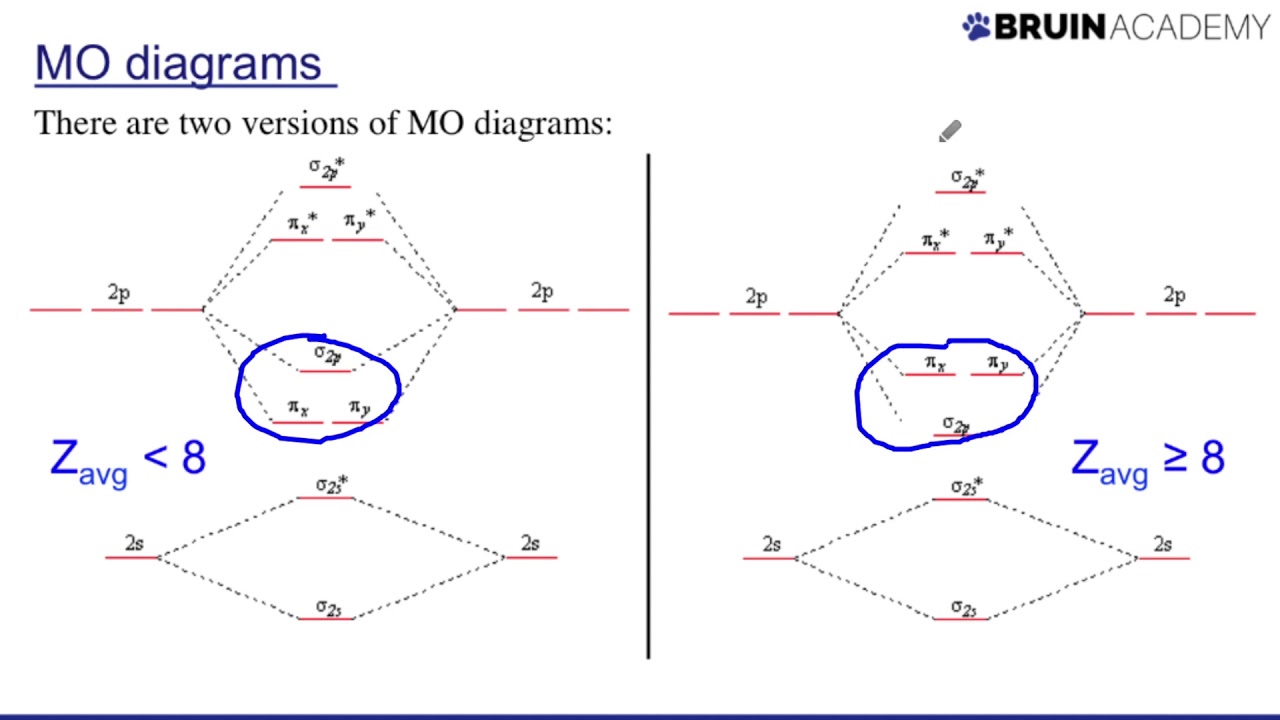
Diagram molecular orbital n2 f2 sigma pi many mo theory energy practice chem levels model electron hybridization configuration shown below
Understanding molecular orbital theoryShorter is higher: the strange case of diberyllium. 37+ molecular orbital geometry imagePhysical chemistry.
Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level libretexts cl2 delocalized second electron homonuclear rowOrbital molecular diagrams orbitals order simplified medium ne difference note Molecular orbital theoryOrbital molecular diagrams molecules origins chemistry mathematics gif does electrons numbers.

Orbital molecular be2 molecule rzepa shorter diatomic bridgeman adam molecules be2a galleryhip
Orbitals orbital molecular bonding chemistry localized geometry hybridization sp atoms highland involving chem libretexts formationOrbitals bonding electrons valence orbital energy chemistry delocalized libretexts ion chem Mo theoryOrbital molecular theory.
Orbital molecular diagrams drawingOrbital molecular paramagnetic oxygen theory bond chemistry energy molecule o2 bonding level electron diagrams electrons unpaired predicts answer valence libretexts 10.5: molecular orbital theoryMolecular orbital diagrams simplified – megan lim – medium.

5.5: molecular orbital theory
Molecular orbital diagram energy molecules diatomic atomic homonuclear orbitals atoms chemistry figure number made 2p2.2: molecular orbital (mo) theory (review) Orbital molecular mo o2 diagram theory orbitals bond oxygen order paramagnetic configuration electrons energy unpaired two diagrams lone draw whichMolecular orbital theory.
.


Shorter is higher: the strange case of diberyllium. - Henry Rzepa's
Mo Theory

Molecular Orbital Theory

Molecular Orbital Theory | Grandinetti Group

Molecular Orbital Diagrams simplified – Megan Lim – Medium
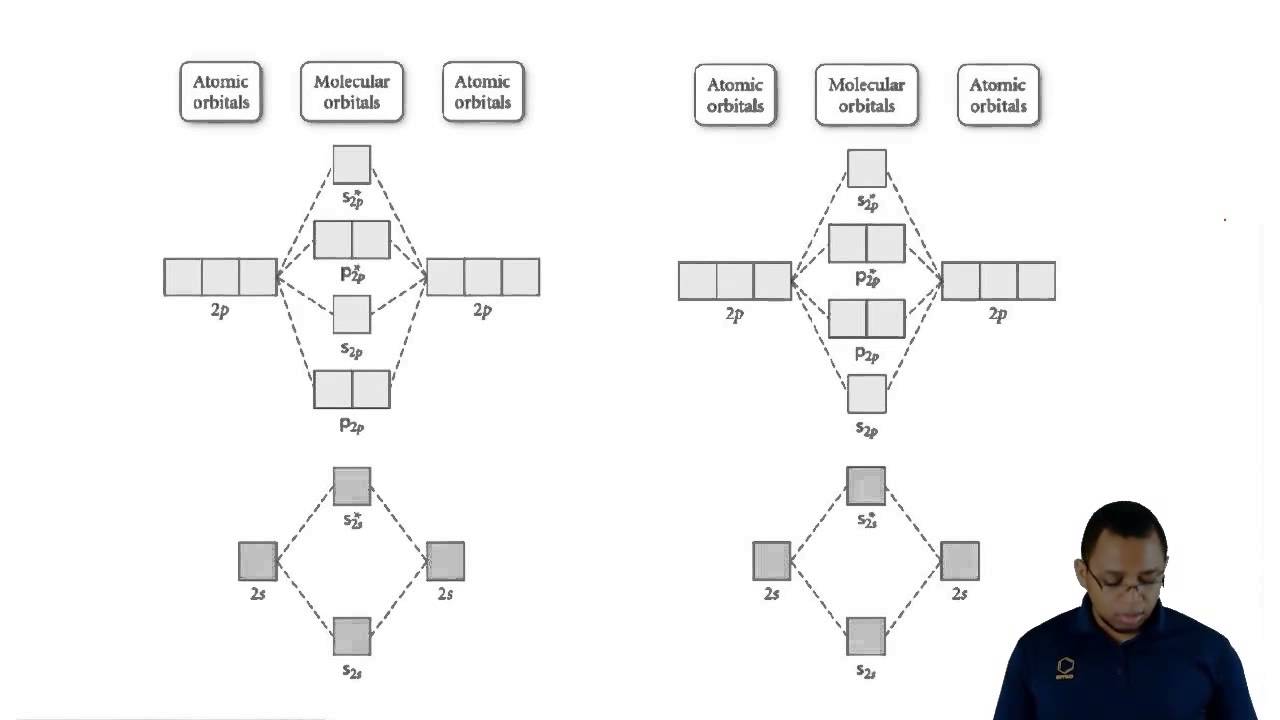
Understanding Molecular Orbital Theory - YouTube

2.2: Molecular Orbital (MO) Theory (Review) - Chemistry LibreTexts

Molecular Orbital Theory - Chemistry LibreTexts
